Manual cell counters, like the hemocytometer, represent the original method for determining cell concentration, requiring a trained scientist and microscope.
These tools are still widely utilized due to their accessibility and foundational role in biological research, offering a direct observation of cells.
What is a Manual Cell Counter?
A manual cell counter is a device used to count cells in a sample, traditionally employing a hemocytometer and a microscope. This method necessitates a trained individual to visually identify and tally cells within a defined area.
Unlike automated systems, manual counting relies on direct observation, offering a hands-on approach to cell quantification. It’s a fundamental technique, particularly valuable when automated options are unavailable or when detailed cell morphology assessment is needed.
Historical Context of Cell Counting
The earliest form of cell counting involved manual techniques, with the hemocytometer becoming a cornerstone in the late 19th century. Initially, researchers lacked automated tools, relying entirely on microscopic observation and painstaking manual tallying.
This foundational method established the principles of cell concentration determination. While automated counters now exist, the hemocytometer remains relevant, representing the historical origin and a cost-effective alternative for many labs.

The Hemocytometer: A Core Component
Hemocytometers are specialized glass slides with precisely etched grids, essential for manual cell counting under a microscope, providing defined areas for analysis.
Hemocytometer Design and Grids
Hemocytometer grids are meticulously engineered with a central chamber divided into nine large squares, each measuring 1mm x 1mm. The central square is further subdivided into 25 medium squares, and each of those into 16 small squares.
These grids facilitate accurate cell counting by providing defined areas; counting cells within these areas allows for concentration calculations. The depth of the chamber is precisely 0.1mm, crucial for volume determination.
Loading and Preparing the Hemocytometer
Preparing the hemocytometer involves thorough cleaning and ensuring the coverslip is securely in place to create a precise chamber depth. Cell suspensions must be properly mixed to ensure even distribution before loading.
A small volume of the sample is carefully introduced under the coverslip via capillary action, allowing it to fill the chamber. Avoiding over or underfilling is critical for accurate counting and reliable results.

Manual Counting Procedure: Step-by-Step
The manual counting process begins with careful cell suspension preparation, followed by focusing the microscope and systematically counting cells within defined grid areas.
Accuracy relies on consistent technique and diligent observation.
Cell Suspension Preparation
Proper cell suspension is crucial for accurate counting. Cells must be thoroughly mixed to ensure even distribution, avoiding clumping which can skew results.
Dilution may be necessary to achieve a suitable concentration for hemocytometer counting, typically aiming for a density where cells are well-separated.
Gentle pipetting is essential to prevent cell damage, and any debris should be removed before loading the hemocytometer chamber.
Focusing and Identifying Cells
Achieving optimal focus is paramount for accurate cell identification. Begin with a low-power objective and gradually increase magnification, carefully adjusting the fine focus knob.
Distinguish between viable and non-viable cells – often based on morphology, such as membrane integrity or granularity.
Clearly define inclusion/exclusion criteria for counting to maintain consistency and minimize subjective bias during the process.
Counting Cells in Defined Areas
Systematically count cells within the hemocytometer’s defined grid areas. Typically, the four corner squares and the central square are counted to minimize edge effects and ensure representative sampling.
Employ a consistent counting pattern – for example, a serpentine or boustrophedon approach – to avoid double-counting or omissions.
Record the number of cells in each counted square meticulously for subsequent concentration calculations.

Calculations and Concentration Determination
Determining cell concentration involves applying a volume correction factor and accounting for dilution. Researchers multiply the cell count by 10,000 to find cells/mL.
Volume Correction Factor
The volume correction factor is crucial for accurate cell counting. A standard hemocytometer has a volume of 0.1 mL, but only a portion is actually counted.
This factor accounts for the specific grid area examined and any dilutions made to the cell suspension. Typically, a factor of 10,000 is used, representing the dilution to achieve cells per milliliter.
Understanding and correctly applying this factor is essential for reliable concentration determination.
Calculating Cells per Milliliter
Calculating cells per milliliter involves multiplying the average cell count from the defined areas by the volume correction factor.
This factor, often 10,000 (104), compensates for the small volume counted within the hemocytometer grid and any initial dilutions.
The resulting value represents the total number of cells present in one milliliter of the original sample, providing a quantifiable measure of cell concentration.

Advantages of Manual Cell Counting
Manual cell counting offers significant cost-effectiveness and accessibility, requiring minimal equipment and providing a straightforward method for cell density assessment.
Cost-Effectiveness
Manual cell counting, particularly utilizing a hemocytometer, presents a remarkably cost-effective solution for cell density determination. Compared to automated systems, the initial investment is substantially lower, requiring only a hemocytometer, microscope, and coverslip.
This makes it ideal for laboratories with limited budgets or those performing cell counts infrequently. The absence of expensive reagents or consumables further contributes to its economic advantage, offering a practical approach to cell quantification.
Accessibility and Simplicity
Manual cell counting boasts exceptional accessibility and simplicity, requiring minimal specialized training beyond basic microscopy skills. The technique is readily deployable in most biological laboratories equipped with a standard microscope and a hemocytometer.
Its straightforward procedure – loading a sample, focusing, and visually counting cells – makes it easily learnable. This contrasts with automated counters needing specific software and maintenance, offering a user-friendly alternative.

Disadvantages of Manual Cell Counting
Manual cell counting is notably time-consuming and susceptible to human error, impacting reproducibility. It demands focused attention and consistent technique for reliable results.
Time Consumption
Manual cell counting inherently requires a significant time investment. Researchers must meticulously prepare cell suspensions, carefully load the hemocytometer, and then painstakingly count cells under a microscope.
Operators often attempt to save time by counting fewer squares on the grid, but this compromises accuracy. Compared to automated systems, which deliver rapid results, manual counting is a considerably slower process, especially when analyzing multiple samples.
Potential for Human Error
Manual cell counting is susceptible to inaccuracies stemming from subjective interpretation and human fatigue. Identifying and differentiating cells can be challenging, leading to miscounts.
Uneven cell distribution within the hemocytometer chamber also introduces variability. While consistent technique and training mitigate these risks, the potential for error remains higher compared to automated counters, which employ objective sensors and algorithms for precise cell enumeration.
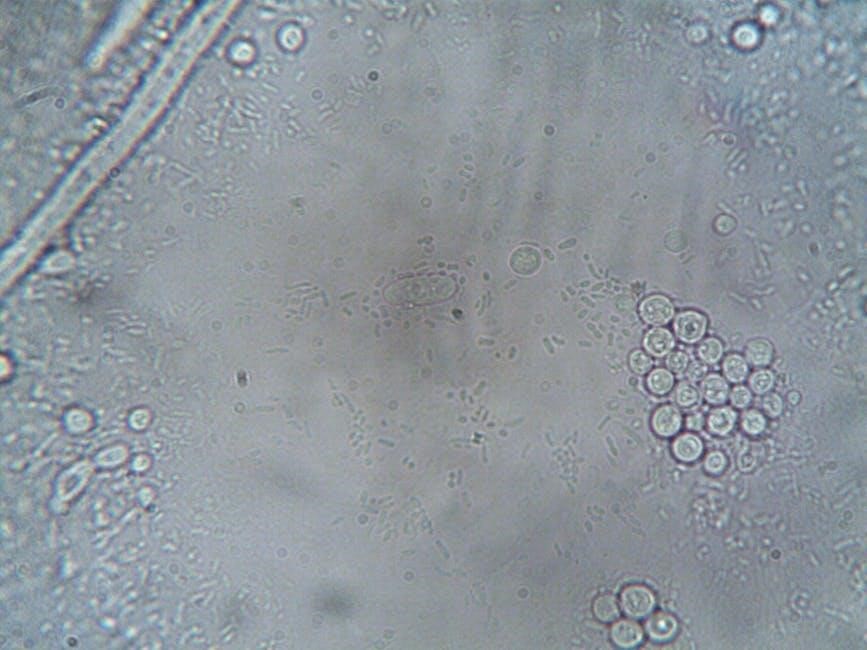
Comparison with Automated Cell Counters
Automated cell counters offer increased throughput and reliability compared to manual methods, utilizing sensors or image analysis for counting.
Manual counting relies on visual assessment, while automated systems minimize subjective error and provide faster results.
Manual vs. Automated: Key Differences
Manual cell counting, traditionally performed with a hemocytometer, necessitates a trained operator to visually count cells under a microscope, making it a labor-intensive process.
Automated cell counters, conversely, employ technologies like image analysis or impedance to count cells without direct human intervention, significantly boosting speed and reducing subjectivity.
While manual methods are cost-effective, automated systems offer higher precision and the ability to analyze larger sample volumes, improving overall data reliability.
Throughput and Reliability
Manual cell counting inherently limits throughput, as each count requires dedicated time and skilled observation; counting only a few squares compromises accuracy.
Automated cell counters dramatically increase throughput by rapidly analyzing numerous samples, interrogating larger areas—like almost four hemocytometer squares—for improved precision.
Automated systems minimize human error and provide consistent results, enhancing reliability, while manual counts are susceptible to subjective interpretation and fatigue.
Applications of Manual Cell Counting
Manual cell counting is crucial for cell culture monitoring and accurately determining yeast cell concentrations, providing essential data for biological experiments and research.
Cell Culture Monitoring
Manual cell counting plays a vital role in cell culture monitoring, allowing researchers to track cell growth and viability over time. Regular counts help optimize culture conditions, ensuring consistent experimental results.
By observing cell density, researchers can determine when to passage cells, preventing overgrowth or nutrient depletion. This technique is fundamental for maintaining healthy and reliable cell lines, crucial for various biological studies and applications.
Yeast Cell Counting
Manual cell counters, specifically hemocytometers, are frequently employed for yeast cell counting in various applications, including fermentation monitoring and quality control. Due to their size, yeast cells are readily observable under a microscope using this method.
Accurate yeast cell counts are essential for determining cell viability and optimizing growth conditions. This technique provides a cost-effective and reliable means of assessing yeast populations in research and industrial settings.

Troubleshooting Common Issues
Manual cell counting can present challenges like uneven cell distribution or difficulty achieving focus. Careful sample preparation and consistent technique mitigate these issues.
Addressing these problems ensures accurate and reliable cell concentration determination.
Uneven Cell Distribution
Uneven cell distribution within the hemocytometer chamber is a frequent issue impacting accuracy. Cells may clump or settle non-uniformly, leading to inconsistent counts across different grid squares.
Gentle, yet thorough, mixing of the cell suspension is crucial before loading. Avoiding vigorous pipetting prevents cell damage, while ensuring adequate dispersion. Counting a larger number of squares, as suggested by automated counter comparisons, minimizes the impact of localized variations in cell density.
Difficulty Focusing
Difficulty focusing on cells within the hemocytometer is a common challenge, particularly at higher magnifications. Proper microscope adjustment, including fine focus and illumination control, is essential.
Ensure the hemocytometer is correctly positioned on the microscope stage and the coverslip is securely in place. Start with low magnification to locate cells, then gradually increase it. Clean optics and adequate lighting significantly improve visibility, aiding accurate cell identification and counting.

Best Practices for Accurate Counting
Consistent technique and thorough proper training are vital for minimizing errors in manual cell counting, ensuring reliable and reproducible results every time.
Consistent Technique
Maintaining a standardized procedure is paramount for accurate manual cell counting. This includes a uniform mixing of the cell suspension before loading, a consistent filling of the hemocytometer chamber, and a systematic approach to selecting counting squares.
Always count the same squares each time, and adhere to a pre-defined rule for including or excluding cells touching the grid lines to minimize bias and ensure repeatability.
Proper Training
Effective manual cell counting necessitates comprehensive training. Individuals must be proficient in preparing cell suspensions, correctly loading the hemocytometer, and accurately identifying cells under a microscope.
Understanding cell morphology, recognizing potential contaminants, and mastering the counting rules are crucial. Consistent practice and supervised sessions are vital to minimize errors and ensure reliable results, ultimately improving data quality.

Future Trends in Cell Counting
Hybrid approaches combining manual observation with software assistance are emerging, aiming to enhance accuracy and efficiency in cell counting workflows.
These innovations seek to bridge the gap between traditional methods and automated systems.
Hybrid Approaches
Hybrid cell counting methods represent a compelling evolution, integrating the strengths of both manual and automated techniques. These systems often utilize microscope images, analyzed with specialized software to aid in cell identification and counting, reducing human error.
This approach allows researchers to leverage their expertise while benefiting from increased throughput and objectivity, offering a balance between cost-effectiveness and precision. Such systems are becoming increasingly popular in labs seeking to optimize their workflows.
Software-Assisted Manual Counting
Software-assisted manual counting bridges the gap between traditional hemocytometer use and fully automated systems. Utilizing microscope-captured images, specialized software helps delineate cells, reducing subjective interpretation and improving accuracy.
These tools can pre-count cells in defined areas, minimizing the time spent manually tallying, and offer features like automated size exclusion to focus on target cell populations. This approach enhances efficiency without sacrificing the visual confirmation of cell morphology.
Resources and Further Learning
Explore relevant publications and online tutorials from Thermo Fisher Scientific and Biology Notes Online to deepen your understanding of manual cell counting techniques.
Relevant Publications
Delving into the literature provides a robust understanding of manual cell counting principles and applications. Researchers should consult publications detailing hemocytometer usage, cell suspension preparation, and accurate counting methodologies.
Specifically, resources from Thermo Fisher Scientific offer practical guidance. Further exploration of peer-reviewed articles focusing on cell biology techniques will enhance proficiency and ensure reliable results in cell concentration determination.
Online Tutorials and Guides
Numerous online resources supplement traditional learning for manual cell counting. Platforms like YouTube host visual demonstrations of hemocytometer loading and cell identification techniques.
Thermo Fisher Scientific provides detailed guides on proper technique and troubleshooting common issues. Interactive tutorials and virtual labs can further refine skills, ensuring accurate cell concentration assessments and minimizing potential errors during the counting process.
Comments